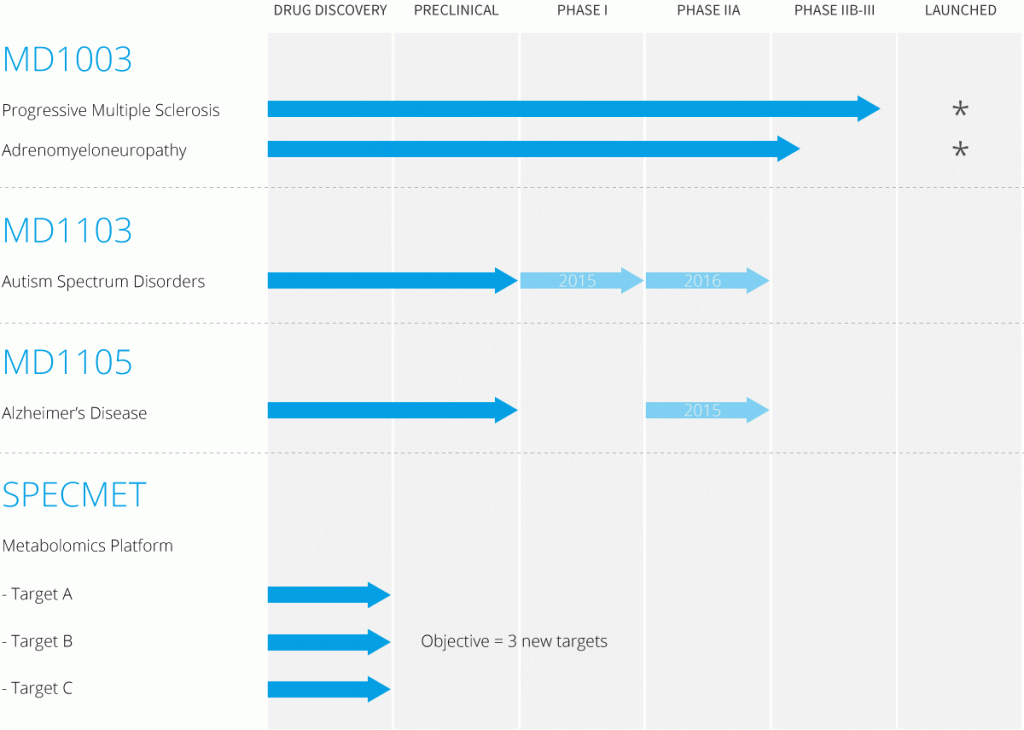
Pipeline
Three molecules are currently in development and SPECMET platform:
- MD1003 in primary and secondary progressive multiple sclerosis (MS) and in X-linked adrenoleukodystrophy
- MD1103 in autism spectrum disorders
- MD1105 in Alzheimer’s disease
- SPECMET platform
MD1003
Progressive Multiple Sclerosis
MD1103

MD1105

SPECMET